Understanding pH Balance
What is pH Balance? We’ll show you!
pH can hold a slightly different meaning depending on the context you are referencing. In this case, we are talking about pH balancing in water chemistry. pH is the measurement of how acidic or basic your water is. The pH scale ranges from 0 to 14, with 0 being the most acidic and 14 the most basic.
pH is the measurement of free hydrogen and hydroxyl ions in the body of water. When referring to the pH scale, if your pH level is 0 and acidic, your water has a greater amount of free hydrogen ions. When your pH level is 14 and basic, that means your water has a greater amount of free hydroxyl.
Ideally, one would have their pH around the neutral mark of 7 and more specifically 7.2-7.6. This is the zone in which our own bodies’ pH levels are set. That being said, it is important to keep your pH balanced and where it should be to ensure comfortable, clear, and well-balanced water.
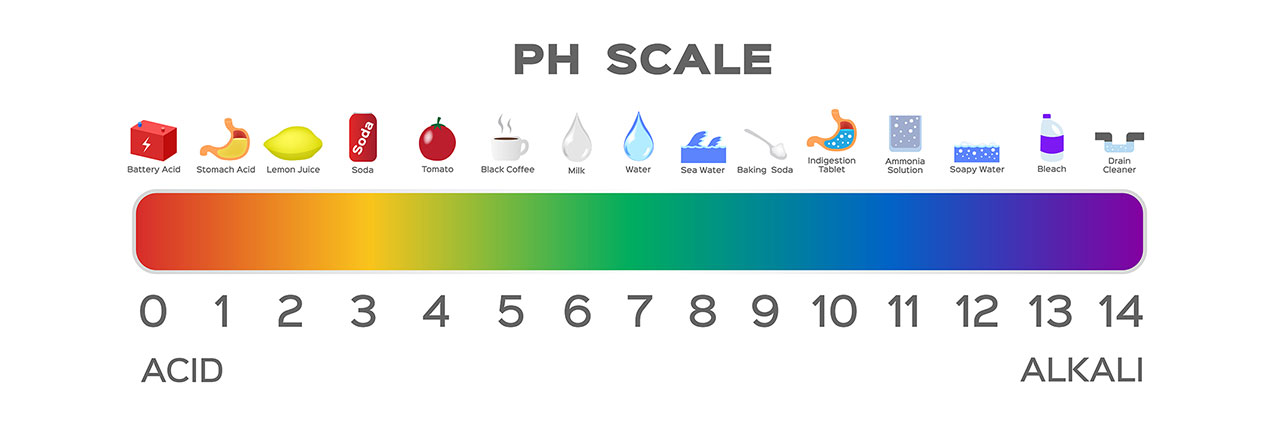
When it comes to balancing your pH, it is important to do so with the correct chemicals and appropriate amounts. If your pH is high, you should add pH decreaser and if your pH is low, you should add pH increaser. Depending on how high or low your pH level is, you will need to add the necessary amount to return the level back to where it should be. You are able to find the dosage for each pH range on the back of the chemical bottle or stop into our store for a water test.
Keep in mind that your pH levels can change very quickly by adding too much increaser or decreaser at once. Because of this, if there is a significant gap between your current pH level and the ideal range for your body of water, you will need to add a small dose of chemical a little bit at a time or a few hours apart.
Keeping your pH balanced is one of the most important aspects of your water chemistry, with the right chemicals and direction, you will always keep your pH in check!

One response to “Understanding pH Balance”